Authors: Aaron Briley, BS; James Clinton, PhD; Brian Shapiro, PhD; Virginia Takahashi, MS; and Chaozhong Zou, PhD
Introduction
Materials and Methods
Results and Discussion
Conclusions
Figures
References
Abstract
Clearance of organic toxins by the kidney is a critical mechanism for mammalian homeostasis and for testing the toxicity of experimental drugs and other compounds. In this study, we created a HEK 293T/17 cell line that stably expresses the human organic anion transporter (OAT1) and tested its transport capabilities.
Download a PDF of this application note
Download NowIntroduction
In vivo studies have shown that kidney membrane transporters play a key part in drug disposition and renal clearance. One such transporter is OAT1 (SLC22A6), which is critical for maintaining the homeostasis of endogenous substances. This makes OAT1 a good transporter to assay for drug interactions in the kidney. Unfortunately, primary cells lose OAT1 expression in culture and transiently expressed OAT1 has great variations between production lots, which makes data hard to interpret. In our study, we generated HEK 293T/17 cells that stably overexpress the OAT1 gene driven by the human elongation factor-1 alpha (EF1α) promoter. We validated that the overexpressed OAT1 transporter has normal transport activities by using 5-carboxyfluorescein (5-CF) and para-aminohipurate (PAH; data not shown) uptake assays and that the uptake can be inhibited by the well-known inhibitors probenecid and novobiocin. Overall, our data has shown that this modified cell line is a very useful in vitro tool for testing the regulation of OAT1 membrane transporter activity in kidney cells.
Materials and methods
HEK 293T/17 (ATCC CRL-11268) cells were transfected with a plasmid expressing the full-length sequence of human OAT1 (NM_004790) under the control of the EF1α promoter via TransfeX Transfection reagent (ATCC ACS-4005). Transfected cells were grown under antibiotic selection and individual positive clones were identified and verified by RT-PCR, immunofluorescence, and Sanger sequencing. A clone negative for OAT1 or the parental line was used as a control. Cell culture medium consisted of DMEM (ATCC 30-2002) supplemented with 10% FBS (ATCC 30-2020). OAT1-HEK transfectants were seeded onto poly-L-ornithine–coated chamber slides and cultured at 37°C/5% CO2. After 24 hours, cells were fixed and stained with a rabbit monoclonal antibody against OAT1 (Abcam) and a goat anti-rabbit 488 secondary antibody then visualized using a fluorescent microscope. Sanger sequencing confirmed that the resulting OAT1 HEK 293T/17 (OAT1-HEK; ATCC CRL-11268G-1) line expressed the complete human OAT1 gene with no mutations (data not shown). Copy number was determined via Droplet Digital PCR (Bio-Rad) to be 6 copies per cell (data not shown).
For the 5-CF uptake assay, OAT1-HEK or control cells were seeded at 105 cells/well in black-walled 96-well plates. After 24 hours, the cells were washed three times in warm HBSS (ATCC 30-2213) and incubated for 10 minutes at 37°C/5% CO2. Cells were then incubated with 150 μM 5-CF (Sigma) for 20 minutes at 37°C/5% CO2. After incubation, the reaction was terminated by washing the cells three times with cold HBSS. Cells were then lysed with M-Per Mammalian Protein Extraction Reagent (Thermo Fisher Scientific) and read immediately on a fluorescent plate reader at 490ex/530em or visualized by fluorescent microscopy. For the inhibition assay, OAT1-HEK cells were incubated with 5-CF and either probenecid (Sigma) or novobiocin (Sigma) for 10 minutes in 96-well plates, then uptake was measured as described above.
Results and discussion
We confirmed the expression of OAT1 in OAT1-HEK by RT-PCR and immunocytochemistry. An approximate 20-fold increase in OAT1 compared to whole kidney lysates was observed. In addition, immunofluorescence revealed that the cells were positive for membrane-localized OAT1 (Figure 1). We then tested the ability of the OAT1-HEK line to uptake a known substrate of OAT1, 5-CF. We observed an uptake ratio of 23:1 relative to the control HEK cells. Moreover, 90% of the OAT1-HEK cells emitted after treatment with the fluorescent 5-CF (Figure 2). The activity of the OAT1-HEK cells was time- and dose-dependent (Figure 3) and was stable over 14 passages (Figure 4). Taken together, these data indicate that the OAT1-HEK cell line stably expressed a functional OAT1 protein.
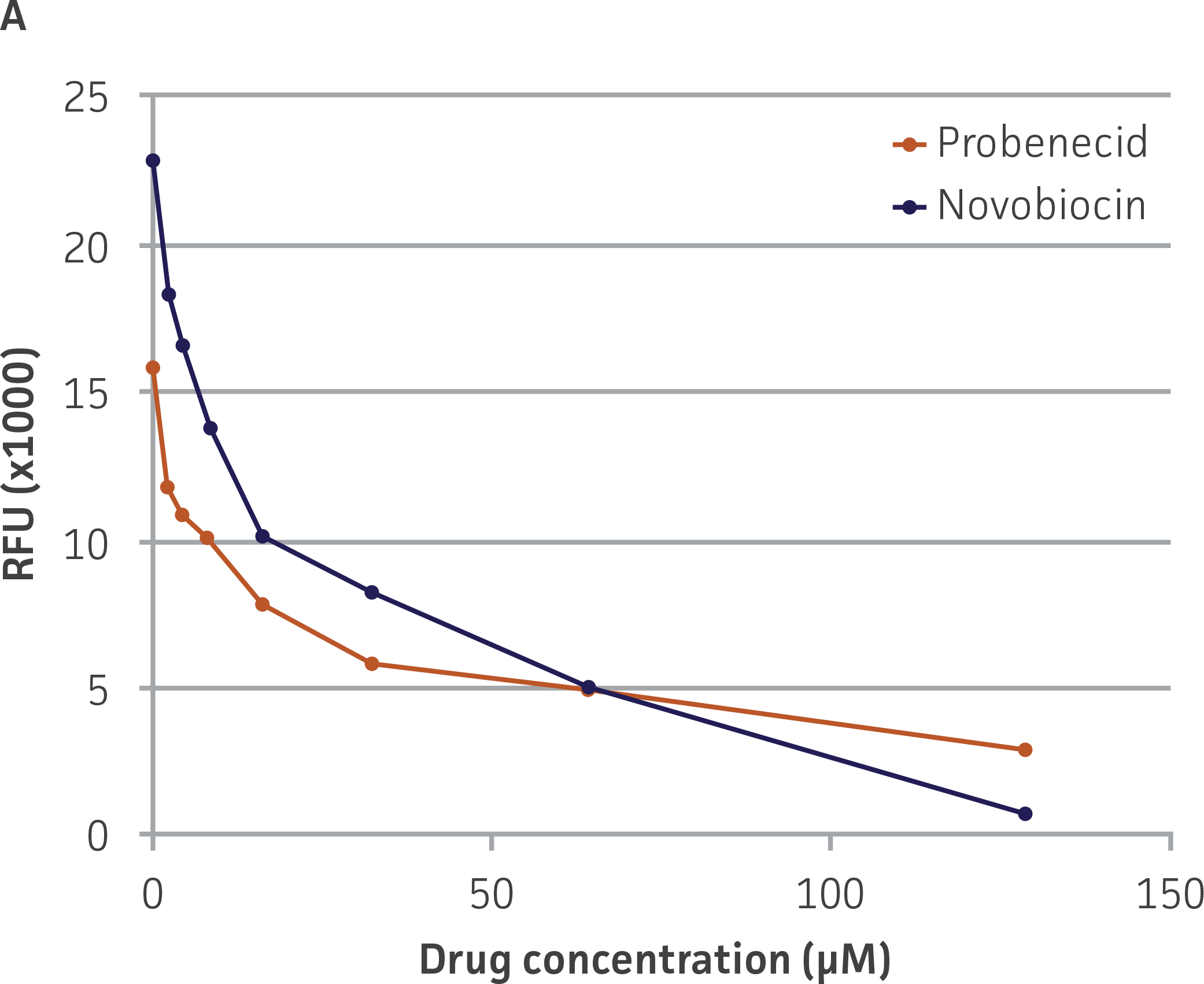
We then tested the ability of two inhibitors of OAT1-mediated activity, probenecid and novobiocin, to block 5-CF transport into the OAT-HEK cells (Figure 5). We observed that both compounds displayed potent inhibitory effects on 5-CF transport (probenecid IC50=15.9 μM; novobiocin IC50=8.1 μM), providing further evidence to the usefulness of these cells in toxicological testing.
Conclusions
We engineered HEK 293T/17 to overexpress the human OAT1 transporter to provide an in vitro model that better mimics the in vivo environment for renal toxicity studies. The OAT1-HEK cell line stably expressed high levels of OAT1 mRNA and membrane-localized protein. We then demonstrated the functionality of the overexpressed OAT by time- and dose-dependent uptake of the fluorogenic substrate 5-CF.
Two known inhibitors of OAT1 activity, probenecid and novobiocin, generated IC50 values similar to those previously reported.1,2 Therefore, OAT1-HEK cells and the 5-CF uptake assay serves as a useful alternative to liquid chromatography-mass spectrometry or radioactive assays for sensitive and high-throughput screening of OAT1 modulators.
Figures
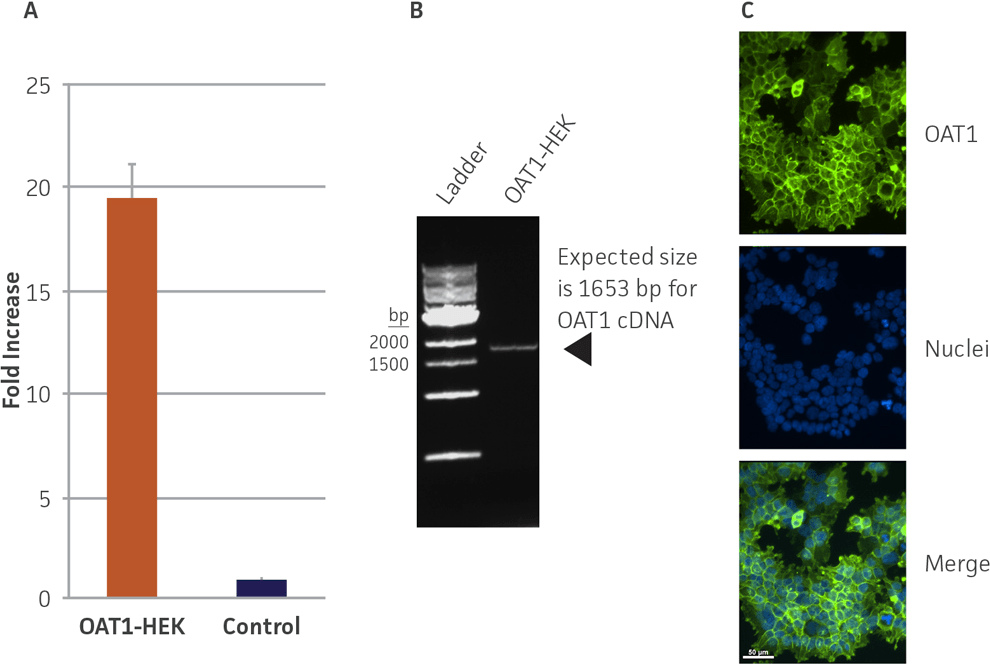
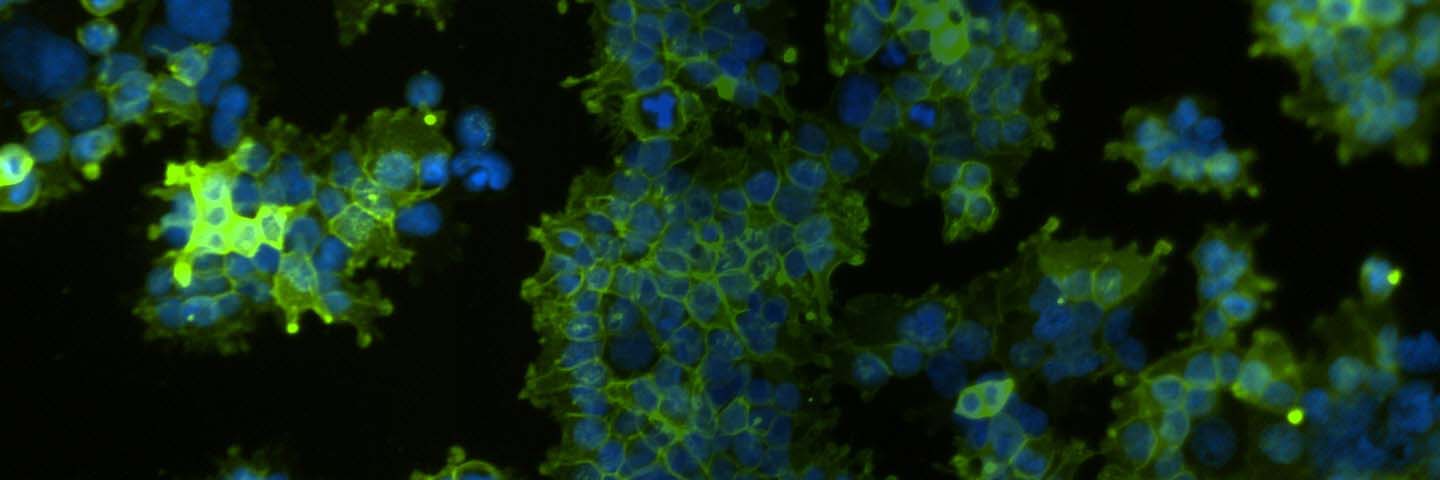
Figure 1. Confirmation of OAT1 expression in OAT1-HEK by RT-PCR and immunocytochemistry. (A) RT-PCR revealed a ~20-fold increase in human OAT1 relative to whole kidney lysate, N=3. (B) The RT-PCR product was analyzed by gel electrophoresis and found to be of the expected size of the human OAT1 sequence. (C) Immunofluorescent staining with a human anti-OAT1 antibody in cultured OAT1-HEK cells revealed the cells were ~90% positive for membrane localized OAT1.
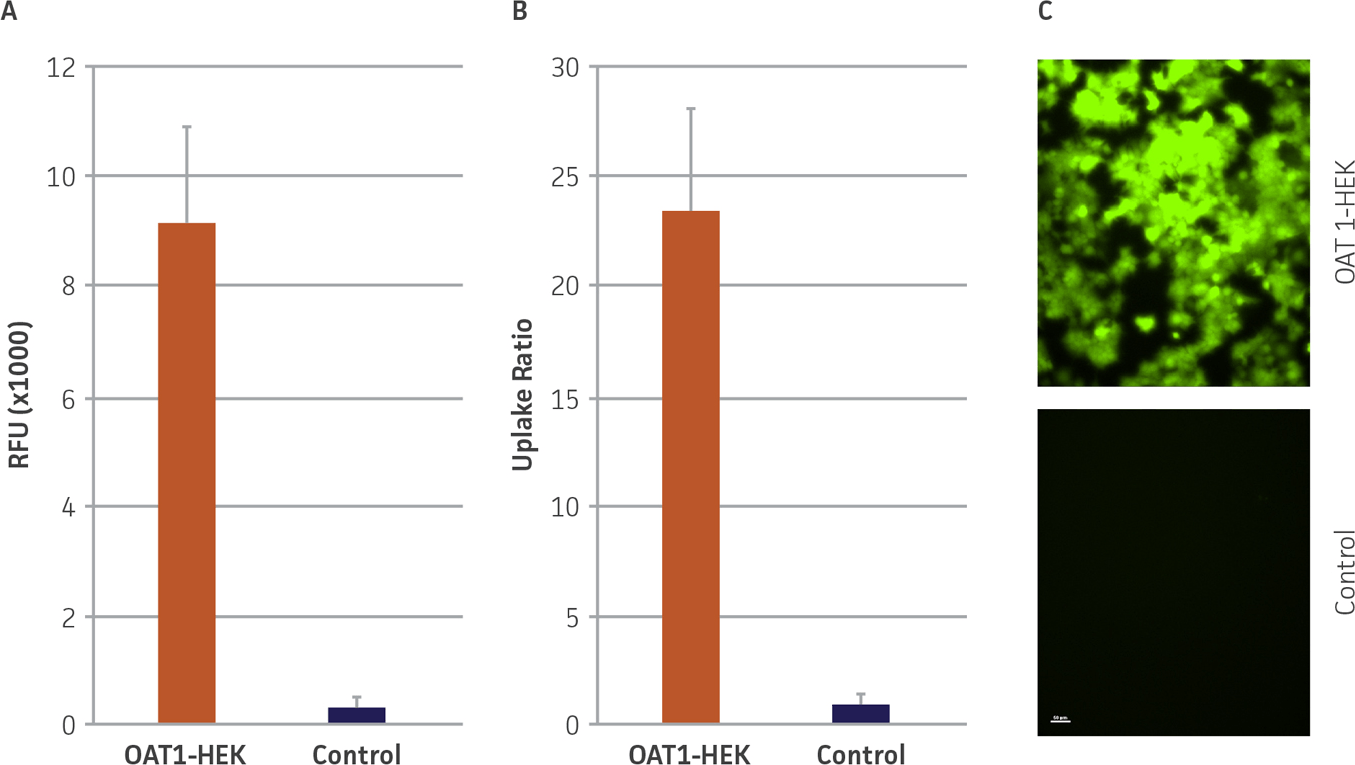
Figure 2. OAT1 mediated uptake of fluorogenic substrate in OAT1-HEK cells. (A) OAT1-HEK or control cells were incubated with the fluorogenic substrate 5-CF as described in the materials and methods. Mean RFUs for the control line was <400 while the signal from the OAT1-HEK line was >9000, N=3. (B) This indicated an uptake ratio of 23 relative to the control, N=3 (middle). (C) Fluorescent microscopy revealed that ~90% of OAT1-HEK, but not control cells, exhibited uptake and accumulation of 5-CF.
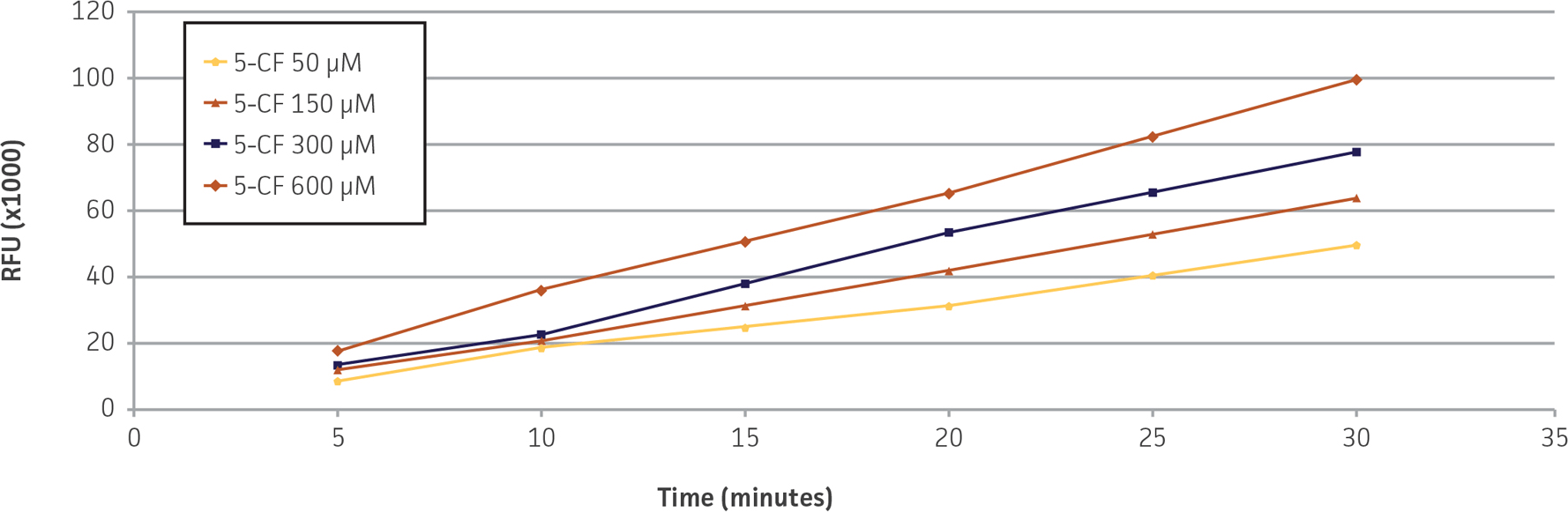
Figure 3. Time- and concentration-dependent uptake of 5-CF in OAT1-HEK cells. OAT1-HEK cells incubated with 5-CF for the indicated times and concentrations demonstrated a linear increase in RFUs (r2 ≥ 0.99) up to 30 minutes, N=4.
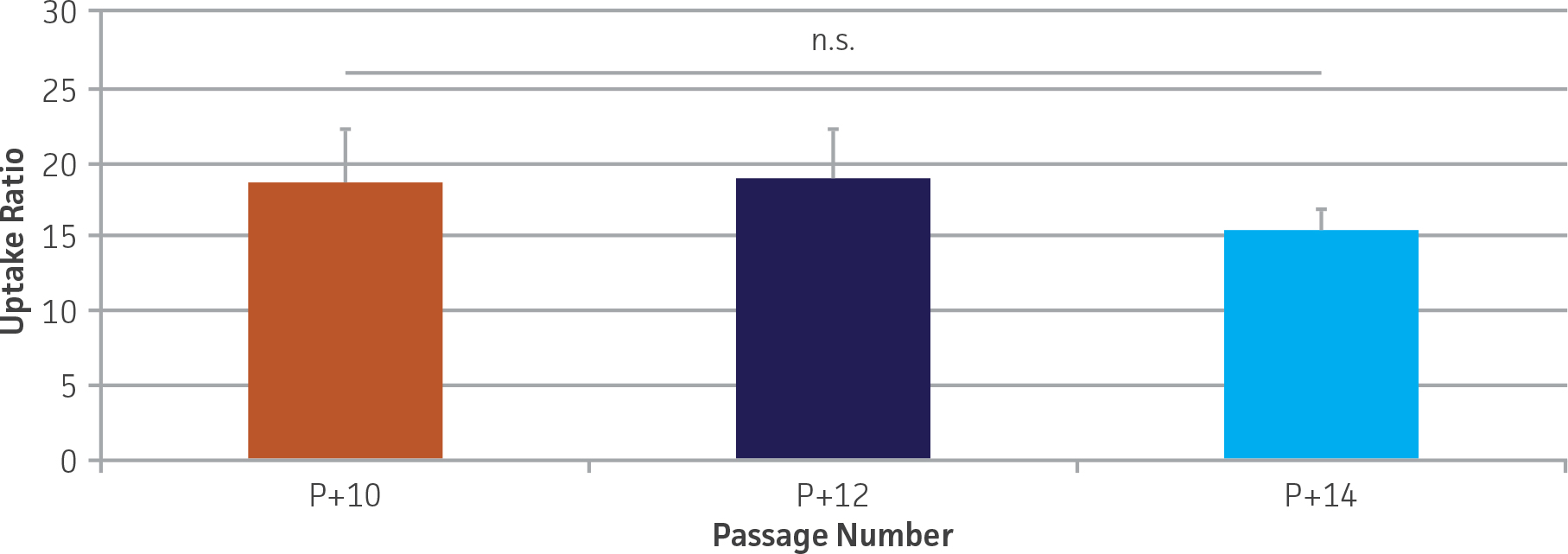
Figure 4. OAT1 mediated 5-CF uptake is stable over extended culture in OAT1-HEK cells. 5-CF uptake was determined as described in the materials and methods and expressed relative to the control line. No significant decrease in uptake was seen up to at least 14 passages (P+ indicates passage number; p=0.77), N=4.
 |
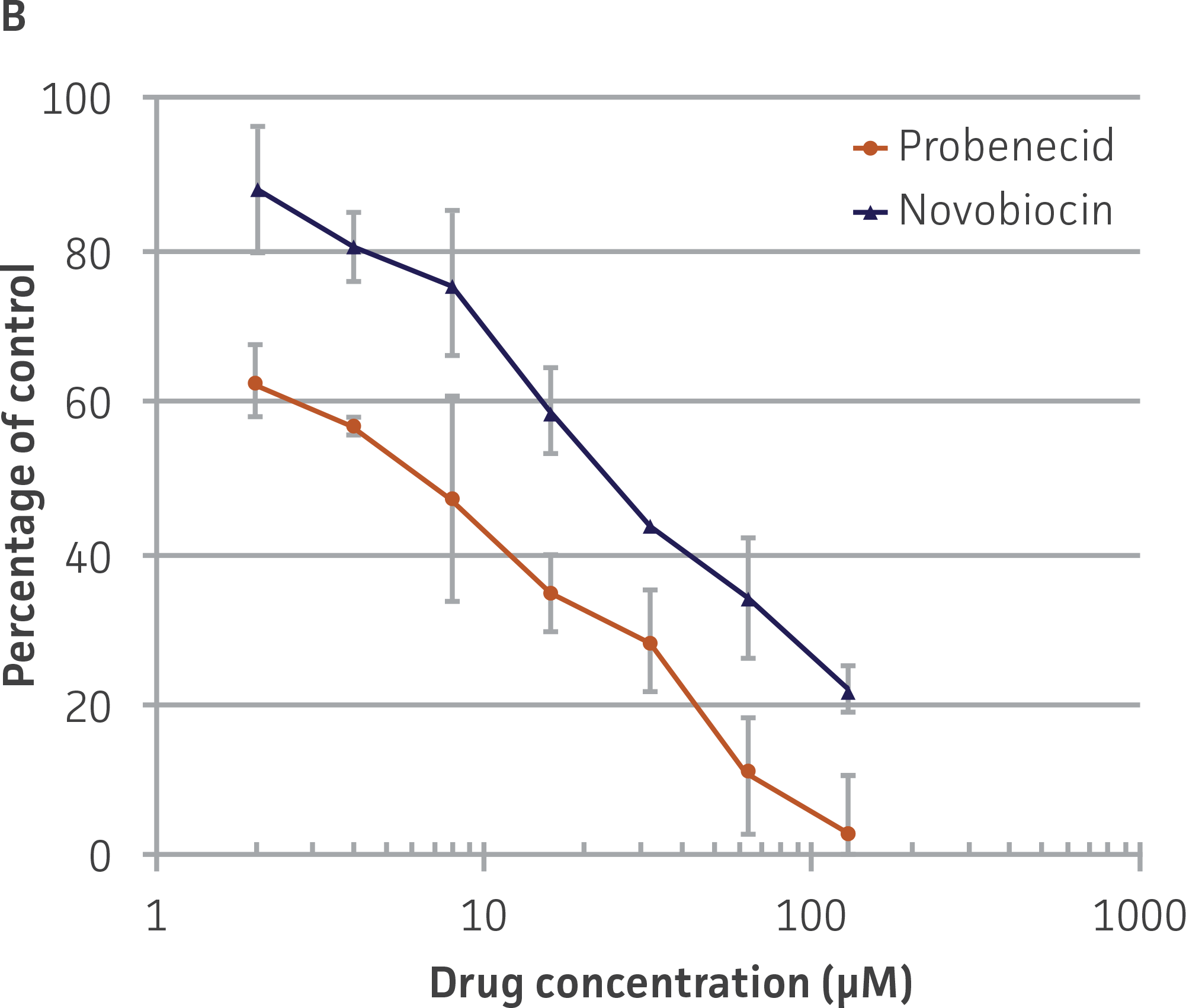 |
Figure 5. Dose-dependent inhibition of 5-CF uptake by OAT1 inhibitors probenecid and novobiocin in OAT1-HEK. OAT1-HEK cells were incubated with the indicated compounds as described in the materials and methods and tested for 5-CF uptake. The results were graphed as (A) raw signal or as (B) percentage uptake relative to non-treated control cells, N=3.
Download a PDF of this application note
Download NowReferences
- Takeda M, et al. Characterization of organic anion transport inhibitors using cells stably expressing human organic anion transporters. Eur J Pharmacol 419(2-3): 113-120, 2001. PubMed: 11426832.
- Duan P, You G. Novobiocin is a potent inhibitor for human organic anion transporters. Drug Metab Dispos 37(6): 1203-1210, 2009. PubMed: 19282394.
