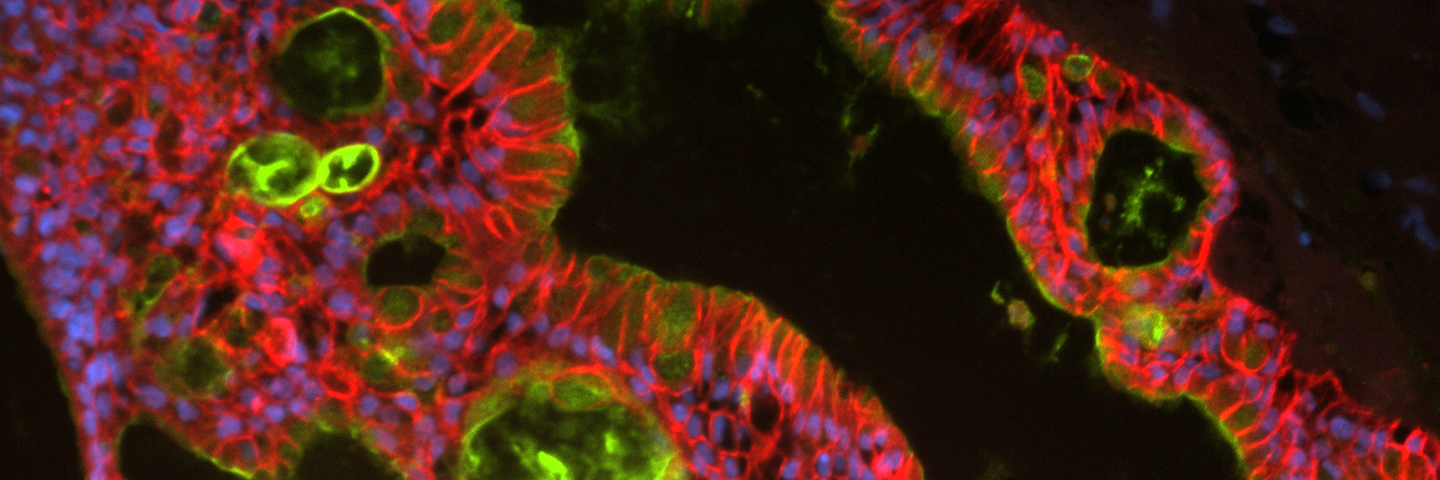
Authors: Allison Ruchinskas, BS, ATCC, and James Clinton, PhD, ATCC
Introduction
Materials and Methods
Thawing from Cryopreservation
Passaging Mature Organoids
Cryopreservation of Mature Organoids
Troubleshooting
Appendix A. Formulation of Complete Medium
References
Abstract
Organoids are complex, self-organizing, three-dimensional in vitro cell culture models that better recapitulate in vivo physiology than traditional two-dimensional culture systems and continuous cell lines. They have many applications in basic and preclinical research, including studies on organogenesis, self-renewal, and the role of the stem cell niche; research on the pathogenesis of cancer and other diseases; and the development and screening of novel drugs. Further, organoids are amenable to many common assays utilized with typical two-dimensional cell culture models such as immunostaining; microscopy, including high-content imaging; and DNA, RNA, and protein analyses. Techniques that are more specialized, such as confocal microscopy or sectioning, may be required to visualize the interior of large organoids that resemble "microtissues." Alternatively, organoids can be disassociated and analyzed by flow cytometry or other methods at the single cell level.
Download a PDF of this application note
Download NowIntroduction
Organoids can be generated from normal or diseased primary tissues of human or animal origin, as well as from induced pluripotent stem cells.1 To date, a number of tissues and organ structures have been recapitulated using organoid model systems (eg, intestine, liver, prostate, breast); these systems exhibit tissue-specific features such as differential cell types and tissue-relevant functions, and they are reported to remain genotypically and phenotypically stable over time.2 The "mini-gut" was one of the first organoid systems to be described3; this model enables the long-term growth and expansion of organoids from the small intestine of mice and humans. Starting with a purified population of stem cells or intestinal crypt fragments isolated from primary tissue, organoids appear within a week after culture and are capable of being rapidly expanded.
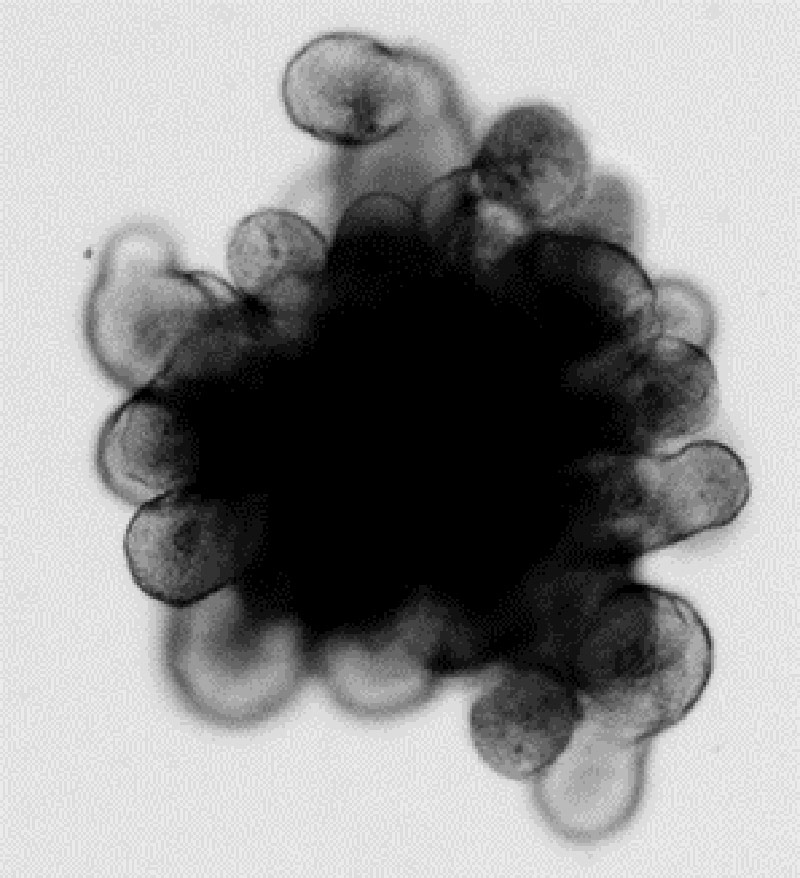
Figure 1. Mouse small intestinal organoid exhibiting a dark central lumen filled with debris and protruding crypt structures.
A key feature of most organoid culture systems is that they require the use of an extracellular matrix (ECM) and specialized, serum-free growth medium containing multiple growth factors and small molecule additives. Organoids are grown embedded within drops or "domes" of solidified, typically undefined, ECM, which allows the organoids to grow in three dimensions (Figure 1 and Figure 2). To expand and continue the culture, organoids are recovered from the ECM domes and are then disassociated into fragments or single cells via enzymatic digestion or mechanical disruption. Intestinal organoids, in particular, exhibit complex morphology with a central lumen from which extensive budding crypts emerge that contain stem cells and differentiated cells (Figure 3 and Figure 4). Here, we describe a protocol for the initiation, culture, and subsequent cryopreservation of mouse small intestinal organoids starting from previously cryopreserved primary tissue-derived organoids.
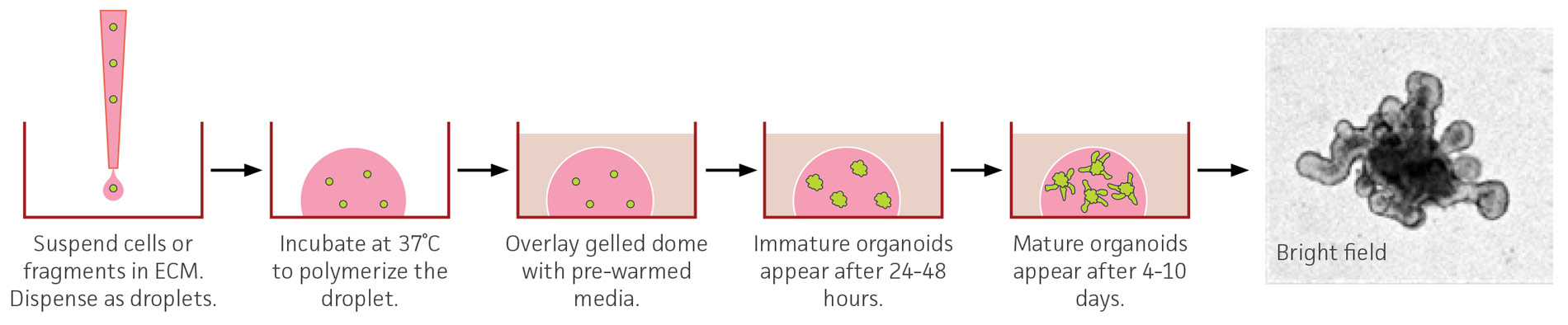
Figure 2. Schematic of a 3-D organoid culture. Overview of an embedded organoid culture system using mouse small intestinal-derived organoids. The bright-field image was captured with a 10X objective and has been enlarged to show organoid morphology and is not to scale.
Materials and methods
Table 1. Required cell culture materials
| ATCC number | |
|---|---|
| DMEM: F-12 Medium (DMEM:F12) | 30-2006 |
| Cell Basement Membrane | ACS-3035 |
| Non-enzymatic Cell Dissociation Solution | 30-2103 |
| ROCK Inhibitor Y-27632 (Y-27632) | ACS-3030 |
| Stem Cell Freezing Medium | ACS-3020 |
| CoolCell LX Alcohol-free Cryopreservation Container | ACS-6000 |
| Complete growth medium (Appendix A) | N/A |
| 6-well tissue culture treated multiwell plates | N/A |
| Bovine serum albumin (BSA) | N/A |
Thawing from cryopreservation
Preparation
- The stock vial of Cell Basement Membrane (5 mL) should be thawed at 4°C and aliquoted. Aliquots should be frozen and stored at -80°C. Once an aliquot is thawed, it should not be refrozen but can be stored at 4°C for at least a week.
Tip: Thawing the stock vial will take several hours; consider thawing it overnight in a refrigerator. - Place a 6-well tissue culture-treated multiwell plate in a 37°C incubator for at least 60 minutes to warm.
- Bring the complete growth medium (Appendix A) to room temperature. Do not warm it in a water bath.
- Thaw the required volume of Cell Basement Membrane on ice. Approximately 100-200 µL is required per well of a 6-well plate.
- Prepare 1% BSA in DMEM:F12 Medium and sterilize it via filtration (0.22 µm). Store it at 4°C.
Thawing of cryopreserved mouse small intestinal organoids
- Thaw the vial rapidly (~2 minutes) in a 37°C water bath. Some ice crystals should remain in the vial.
- Transfer the vial contents to a 15 mL conical tube containing 9 mL of cold 1% BSA in DMEM:F12.
- Rinse the inside of the vial with 1 mL of cold 1% BSA in DMEM:F12 and transfer the medium to the same conical tube containing 9 mL of cold 1% BSA in DMEM:F12.
- Centrifuge the conical tube at 300 x g at 4°C for 5 minutes.
- Carefully aspirate the supernatant without disturbing the pellet.
- Re-suspend the pellet in undiluted Cell Basement (pipet up and down 10 times).
- Dispense approximately 8-10 droplets (10-15 µL per droplet) into each well of the pre-warmed 6-well plate (Figure 2). Avoid creating bubbles. The droplets should retain their shape.
Note: If the droplets flatten and spread out to coat the well, it is possible that the plate was not warm enough or the droplets were not dispensed properly. - Place the plate containing the droplets in a 37°C, 5% CO2 humid incubator for 15 minutes to polymerize the droplets.
Tip: The plate can be inverted while in the incubator to facilitate the formation of domes. - Add 2 mL of the complete growth medium supplemented with 10 µM Y-27632 to each well.
Note: The omission of Y-27632 will significantly reduce post-thaw recovery. After 24 hours in culture, Y-27632 is no longer required in the medium. - Return the plate to the cell culture incubator.
- Perform a complete medium change every 2-3 days.

Figure 3. Appearance of properly dispensed Cell Basement domes. One well of a 6-well plate containing eight cell basement domes after polymerization and before overlay with medium
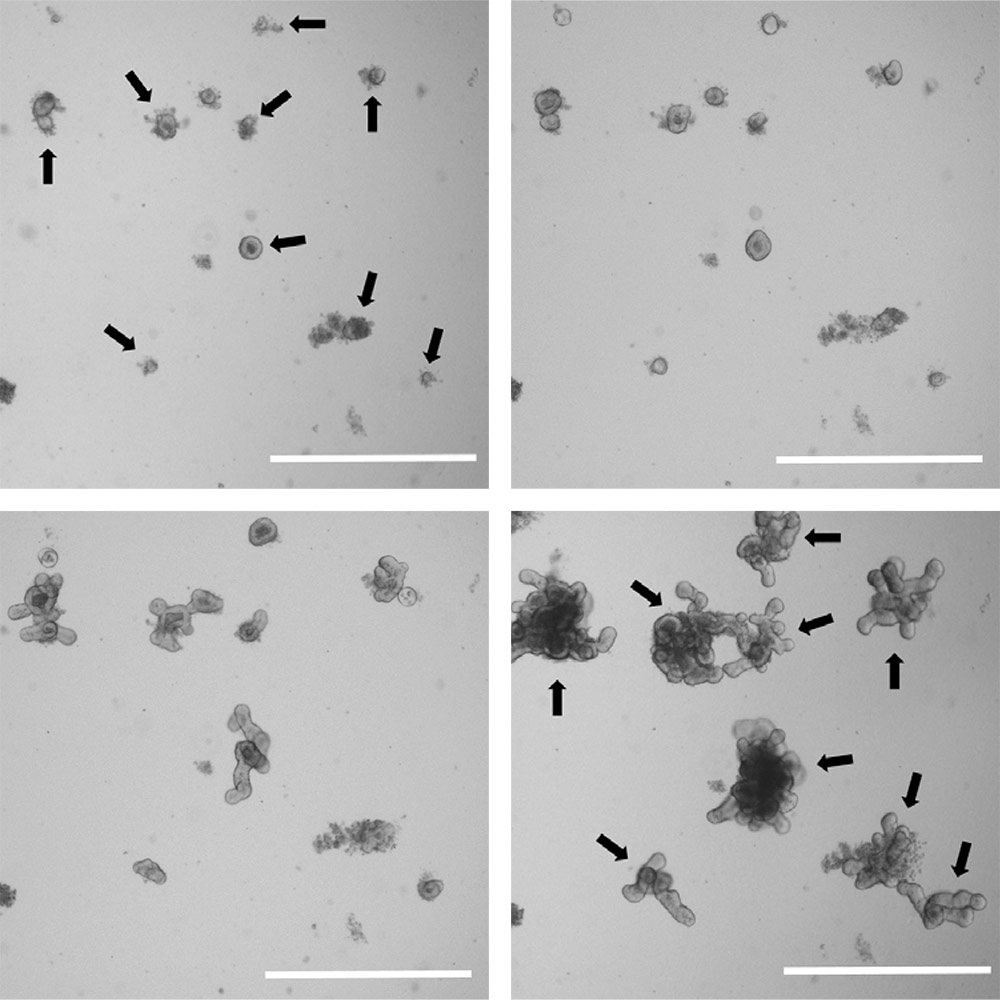
Figure 4. Organoids grow rapidly in size and complexity. Brightfield images of a low density organoid culture at 1 day (upper left), 2 days (upper right), 4 days (lower left), and 7 days post passage (lower right). Individual organoids are indicated by arrows. Scale bar is 1000 µm.
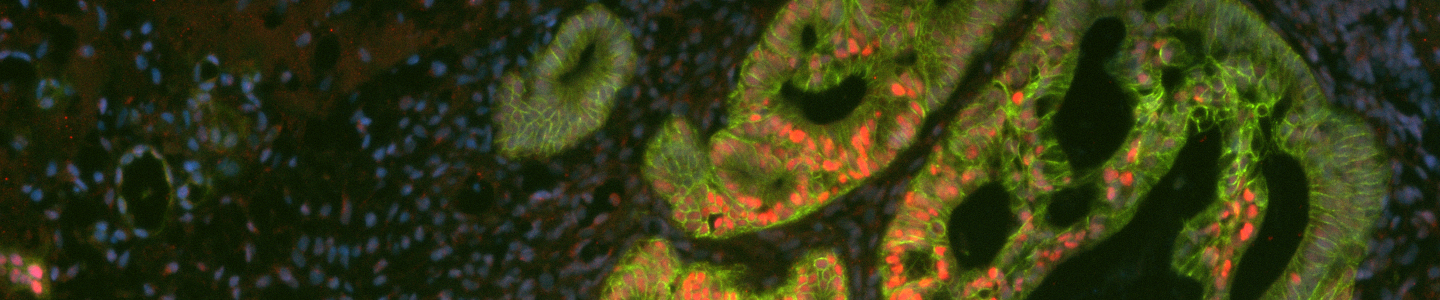
Passaging mature organoids
Preparation
- The stock vial of Cell Membrane Basement (5 mL) should be thawed at 4°C and aliquoted. Once an aliquot is thawed, it should not be refrozen but can be stored at 4°C for at least a week.
Tip: Thawing the stock vial will take several hours; consider thawing it overnight in a refrigerator. - Place a 6-well tissue culture-treated multiwell plate in a 37°C incubator for at least 60 minutes to warm.
- Bring the complete growth medium (Appendix A) to room temperature. Do not warm it in a water bath.
- Thaw the required volume of Cell Basement Membrane on ice. Approximately 100-200 µL is required per well of a 6-well plate.
- Prepare 1% BSA in DMEM:F12 Medium and sterilize it via filtration (0.22 µm). Store it at 4°C.
Passaging
- Organoids can be passaged once they are mature, exhibiting a dark central lumen and multiple crypt domains. Typically, this is 7-10 days post seeding.
- Completely aspirate the medium from the selected wells. Organoids from up to 3 wells can be pooled and processed simultaneously.
- Add 2 mL of cold Non-enzymatic Cell Dissociation Solution per well.
- Incubate the plate for 1 minute at room temperature.
- Use a cell scraper to release the domes from the plate.
- Pre-rinse a P1000 tip with Non-enzymatic Cell Dissociation Solution and then break up the domes by pipetting up and down 10 times.
- Transfer the fragmented domes to a 15 mL conical tube.
- Place the conical tube on an orbital shaker or rocker for 15-20 minutes at room temperature at a low speed.
- Add a sufficient volume of ice cold 1% BSA in DMEM:F12 to bring the total volume to 13 mL.
- Spin the conical tube at 300 x g for 5 minutes at 4°C.
- Carefully aspirate and discard the supernatant; do not disturb the pellet.
- Re-suspend the pellet in 13 mL of ice cold 1% BSA in DMEM:F12.
- Spin the conical tube at 300 x g for 5 minutes at 4°C.
- Carefully aspirate and discard the supernatant; do not disturb the pellet.
- Re-suspend the organoids in undiluted Cell Basement Membrane. Pipet up and down 20 times.
Note: The volume of Cell Basement Membrane will depend on your desired split ratio. We routinely split organoids at 1:6. - Dispense approximately 8-10 droplets (10-15 µL per droplet) into each well of the pre-warmed 6-well plate. Avoid bubbles.
- Invert the plate and place it in 37°C humid incubator for 15 minutes.
- Add 2 mL of the complete growth medium per well.
- Return the plate to the incubator.
- Perform a complete media change every 2-3 days with pre-warmed media.
Note: Only warm the volume of media necessary to perform the media change.

Figure 5. Appearance of organoid culture post-passage and after culture. Mouse small intestinal organoids growing within a single dome of Cell Basement Membrane at day 1 (left) and day 7 post passage (right). Scale bar is 1000 µm.
Cryopreservation of mature organoids
- Aspirate media from wells containing the organoids that need to be cryopreserved. Organoids from up to 3 wells can be pooled and processed simultaneously.
Tip: Large organoids with extensive budding will recover best from cryopreservation. Typically, mouse small intestinal organoids in culture for 7-10 days work best. Approximately 250-500 organoids should be cryopreserved per vial or 1 well of a 6-well plate. - Add 2 mL of cold Non-enzymatic Cell Dissociation Solution per well.
- Incubate the plate for 1 minute at room temperature.
- Use a cell scraper to release the organoids from the plate.
- Pre-rinse a P1000 tip with Non-enzymatic Cell Dissociation Solution and then break up the domes by pipetting up and down 10 times.
Note: Domes and organoids should be broken into small fragments. Do not leave the organoids in Non-enzymatic Cell Dissociation Solution for extended periods or pipet excessively. - Transfer the fragmented domes to a 15 mL conical tube.
- Add a sufficient volume of cold 1% BSA in DMEM:F12 to obtain a total volume of 13 mL.
- Centrifuge the conical tube at 300 x g for 5 minutes at 4°C.
- Carefully aspirate and discard the supernatant; do not disturb the pellet.
- Re-suspend the pellet in undiluted Stem Cell Freezing Medium at approximately 1 mL per well of a 6-well plate.
- Transfer 1 mL of solution to each cryovial.
- Place the cryovials in a CoolCell LX Alcohol-free Cryopreservation Container and transfer the container to a -80°C freezer for 8-24h.
- Remove the cryovials from the CoolCell LX Alcohol-free Cryopreservation Container; transfer the cryovials to the vapor phase of liquid nitrogen for long-term storage.
Troubleshooting
| Problem | Potential cause(s) and solution(s) |
|---|---|
| Low post-thaw recovery | It is not unusual for the initial post-thaw recovery of organoids to be low (<50%); however, if it is significantly lower than expected, ensure that Y-27632 was included in the growth medium during the first 24h post-thaw. If recovery is still low, Y-27632 can be kept in the media for an additional 2-3 days to further promote recovery. |
| Slow growth post-thaw | Thawed organoids may initially grow slowly; typically growth increases after the first passage and 7-10 days in culture. |
| Few or no organoids develop post-passage | During passaging, it is critical that the organoids are not broken up too small or else the organoid formation efficiency will be drastically reduced. The goal should be to break each organoid into multiple fragments, not to reduce organoids to the single cell level. |
| ECM does not form domes | Ensure the multiwell plate has been pre-warmed prior to dispensing Cell Basement Membrane into the plate. Ensure the Cell Basement Membrane is at or near room temperature before dispensing onto warmed plate. |
Download a PDF of this application note
Download NowAppendix A. Formulation of complete medium
| Component | Final concentration | Source |
|---|---|---|
| Advanced DMEM/F12 | - | Thermo Fisher Scientific |
| L-Glutamine (ATCC 30-2214) | 1X | ATCC |
| N2 Supplement | 1X | Thermo Fisher Scientific |
| B27 Supplement | 1X | Thermo Fisher Scientific |
| N-acetyl cysteine | 1 µM | Sigma |
| HEPES | 10 mM | Sigma |
| Epidermal growth factor | 50 ng/mL | R&D Systems |
| Recombinant Noggin | 100 ng/mL | R&D Systems |
| Recombinant R-spondin-1 | 1 µg/mL | R&D Systems |
Formulation adapted from Sato et al. 2011.
References
- Dutta D, et al. Disease modeling in stem cell derived 3D organoid system. TrendsMol Med 23(5): 393-410, 2017. PubMed: 28341301
- Sato T, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141(5): 1762-1772, 2011. PubMed: 21889923
- Sato T, et al. Single Lgr5 stem cells build crypt villus structures in vitro without a mesenchymal niche. Nature 459(7244): 262-265, 2009. PubMed: 19329995
