EMT and MET reporter cell lines: Elevating biological models of metastasis
October 24, 2019, at 12:00 PM ETAbstract
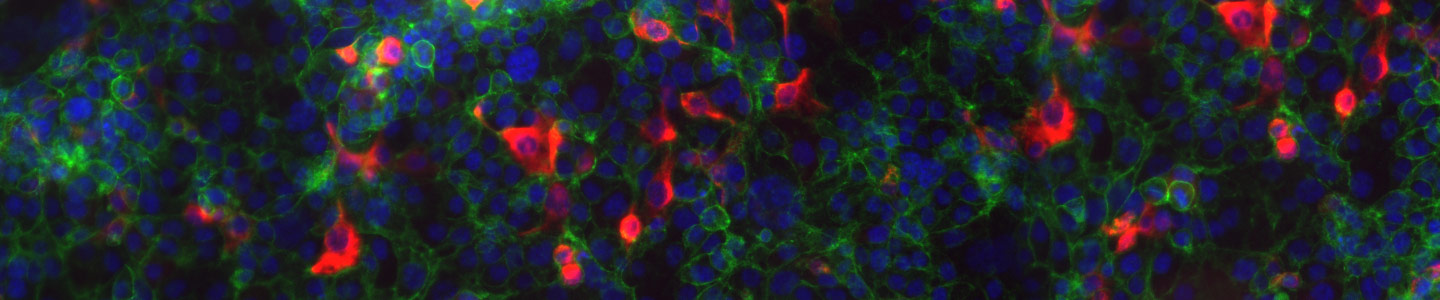
Epithelial-to-mesenchymal transition (EMT) and its reverse (MET) are physiological mechanisms implicated in cancer metastasis. To provide researchers with advanced biological models to study these phenomena, ATCC scientists used their mastery of CRISPR/Cas9 technology to create a number of gene-edited EMT and MET reporter cell lines. These reporter lines enable real-time monitoring of the transition of cells from epithelial to mesenchymal state (or the reverse) via the expression of red fluorescent protein (RFP)–tagged vimentin or green fluorescent protein (GFP)–tagged E-cadherin. This webinar will provide an overview of ATCC’s EMT/MET models and highlight the compelling transition data that was revealed in their development and validation.
Key Points
- CRISPR/Cas9 genome-editing technology was applied to develop a number of RFP- or GFP-tagged reporter cell lines to study EMT and MET phenomena.
- The EMT/MET reporter cell lines can be used to monitor cellular changes in real time or as a platform for drug screening.
- In depth transition data were generated including morphology change, intrinsic reporter expression, marker expression, and invasion upon stimulation with EMT/MET agonists.
Presenter
Diana Douglas, BS
Senior Biologist, ATCC
Diana Douglas is a Senior Biologist at ATCC. For the last four years, she has focused her research on the development of advanced biological models via CRISPR/Cas9 gene-editing technology. Previously, Ms. Douglas worked at the Baker Institute for Animal Health at Cornell University and the Dalton Cardiovascular Research Center at the University of Missouri, where her research focused on the mechanisms of necrotic cell death in heart disease. Ms. Douglas attended Truman State University where she obtained a Bachelor of Science in Biology.
Questions and Answers
Can you also use TGF beta1 to induce EMT?
Yes, we also used TGF beta 1 to induce EMT. Both R&D systems EMT inducing reagent and TGFbeta1 able to induce cells.
Is there any difference between the cell culture medium/method used for the EMT line and the parental line?
We used the same media to culture both EMT and parental line.
Do you check if the E-cadherin or vimentin expression in knock-in cells is similar to the parental cells?
We do check by doing Immunocytochemistry if the Ecadherin in parental and gene edited cells are similar.
Do these EMT reporter cell lines have any un-desired mutations (indels) caused by CRISPR gene editing?
We did analysis on 10 potential most probable off target sites for the gRNA that we used to create GFP reporter KI at Ecadherin locus. Amplified those off target sites and sequenced all of them. We did not see any indel on those off-target site that could have been caused by CRISPR/Cas9 gene editing.
Can you tell us what did you use to induce EMT in MCF 10AEMT reporter line?
We used StemXVivo EMT Inducing Media Supplement from R&D systems to induce EMT and it takes 5 days to induce EMT using this reagent.
Does MCF 10AEMT reporter line contain any antibiotic gene sequences at the E-cadherin locus?
No, reporter line does not contain any antibiotic gene at Ecadherin locus.
Do the cell lines have single allele knock-in or double alleles knock-in?
Based on our RT-PCR data, KI allele seems to be homozygous.
What is the main application of MCF 10A EMT or other EMT cell lines?
MCF 10AEMT cells displayed significant morphology changes and showed a decrease in ECAD-EmGFP expression upon EMT induction. Therefore, this cell line can be used to track the EMT status in live cells in real time by monitoring GFP expression. Additional applications may include the high-throughput (HTS) discovery of new agents targeting EMT/MET.
