Drug discovery and development has a long, rich history spanning from the folk remedies generated during the early days of human civilization to the booming pharmaceutical industry we know today. Throughout this time, drugs evolved from plant- and animal-based medicines to synthetically derived compounds that have since drastically altered medical practices and the ability of patients to overcome and survive disease. With the continuing advancements in biotechnology and our expanding knowledge of the human genome, researchers are now able to identify the underlying causes of a disease and use this information to develop drugs directed toward specific targets.
Resources for screening
High-throughput Screening
Discover more on the development of cell-based high-throughput screening assays for drug development and how to choose a relevant cell model.
Toxicological screening
Toxicity testing of new substances is an essential step in the drug development process. Discover how advanced cell models like differentiated iPSCs and hTERT-immortalized primary cells can help.
The drug development process
The contemporary drug development process is an arduous one, often requiring 10-15 years and more than $2 billion for the design, development, and approval of one new drug for use. Before a drug is approved for patient use, it must undergo rigorous testing to ensure that it is safe and effective against the intended target. This process begins with the identification of a drug target and the testing of thousands of chemical compounds in the drug discovery stage. This essential stage comprises several core steps:
- Target identification and validation – Two common reasons why drugs fail during clinical trials is because they are not effective or they are unsafe. Therefore, having a clear understanding of the disease and what underlying factors contribute to that disease is essential when identifying potential drug targets (i.e., proteins, genes, RNA, or cellular structures). The target chosen should be safe and efficacious, and its role in the disease should be clearly defined. A good drug target also needs to be “druggable” in the sense that it is both accessible to a drug and the desired response is elicited following the binding of the drug to the target. Once a target is identified, it must be further validated to demonstrate that it is involved in the disease process and that its modulation will likely have a therapeutic effect.
- Hit identification and validation – Once a target has been validated, screening approaches can be used to identify “hit” compounds that interact with the target of interest. There are several strategies that can be used to identify hits such as high-throughput screening, fragment screening, virtual screening, and phenotypic screening. Depending on the nature of the project, one or more of these approaches can be used.
- Hit-to-lead – After the hit series have been identified, the list of hits is further refined to those that demonstrate promising therapeutic effects. Here, secondary assays are employed to screen hits for off-target effects; assess solubility and permeability; and evaluate physiochemical and absorption, distribution, metabolism, and excretion (ADME) properties.
- Lead optimization – Following lead selection, the compounds are chemically modified to create analogs that demonstrate improved potency, reduced off-target activity, and optimized pharmacokinetic properties. The highest quality leads that may be suitable for preclinical or clinical testing are then selected as drug candidates; these compounds must be able to interact with the target and elicit the desired response while not being toxic.
The extensive costs in time, money, and effort needed to bring a new drug to market underscore the importance of optimizing the drug discovery phase to ensure that only the most effective and safe drug candidates are chosen for pre-clinical testing. To support the different stages of this process, researchers need access to physiologically relevant cell models, clinically relevant microorganisms, first-in-class biomaterial storage, and reference-quality data. That’s why ATCC has made it a priority to provide the tools needed to investigate the efficacy and safety of new chemical entities. Explore our resources below to discover more.
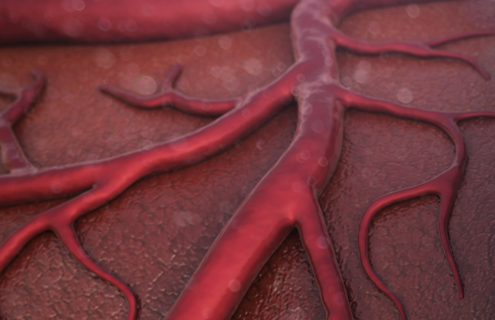
Angiogenesis
While the formation of new blood vessels is a normal part of development, growth, and repair, it also plays a role in diseases like cancer. Tumors need nutrients and oxygen to grow and spread, so they secrete vascular growth factors to stimulate angiogenesis to cause excessive blood vessels to grow into the tumor. To tackle this issue, clinicians have turned toward angiogenesis inhibitors to treat certain cancers.
Learn MoreAntimicrobial Resistance
The spread of antimicrobial resistance has threatened our ability to treat even the most common of infections. As this global health threat grows, it is now more important than ever that researchers develop faster diagnostic tests and discover new vaccines and treatments that are effective against antimicrobial-resistant pathogens. Explore the innovative approaches that have emerged.
Explore Now