MPS technology offers several advantages over traditional cell culture or animal testing methods. It provides a more accurate and physiologically relevant representation of human organs and tissues. MPS platforms also offer a realistic and dynamic setting for studying cellular responses and behaviors by recreating the organ-specific microenvironment, cellular interactions, and mechanical forces.5
Because of these advantages, MPS have the benefit of better predicting and evaluating drug responses in various human organs, which is a limitation of the traditional toxicity testing methods that rely on animal models.6 MPS can detect subtle toxic effects that may not be observed in animals, leading to a more comprehensive understanding of drug safety profiles.7 MPS models allow researchers to screen potential drug candidates and assess their safety and efficacy in a more rapid and cost-effective manner than traditional approaches.8 By simulating the absorption, distribution, metabolism, and excretion of drugs, MPS platforms can help identify promising lead compounds and reduce the reliance on animal models. It also helps mitigate risks associated with drug-induced side effects and adverse reactions in the early stages of drug development.9
MPS also offer unique opportunities for disease modeling. By recreating the structure and function of organs affected by diseases, researchers can study disease progression, evaluate potential therapeutic interventions, and gain insights into disease mechanisms.10 MPS platforms have been successfully utilized to model various conditions, including cancer,11 neurodegenerative diseases,12 cardiovascular disorders,13 and infectious diseases.14
Additionally, these systems enable the testing of combinations of drugs or personalizing medicine. Personalized medicine is an emerging field that aims to tailor medical treatments to individual patients based on their genetic makeup. MPS technology plays a crucial role in this area by incorporating patient-specific cells or induced pluripotent stem cells. By using cells originating from the patient, MPS platforms provide a personalized approach to drug testing and treatment. This allows for the evaluation of individual responses to various drugs, enhancing the effectiveness and safety of treatments.15
In conclusion, MPS are transforming the field of biomedical research. These innovative platforms offer more accurate and physiologically relevant models of human organs and tissues, allowing for accelerated drug discovery, improved toxicity testing, and enhanced disease modeling. With further advancements in technology and research, MPS have the potential to revolutionize personalized medicine and improve patient outcomes.
ATCC has established an MPS program with the mission of supporting the scientific community in this field by providing characterized and validated biologicals and aiding the standardization of protocols in various MPS technologies. Further, we are collaborating with technology developers to accelerate product development.
Did you know?
ATCC has over 200 organoids representing 15 different tissue types.
Meet the author
Carolina Lucchesi, PhD
Principal Scientist, BioNexus, ATCC
Carolina Lucchesi is BioNexus Foundation Principal Scientist leading the Microphysiological Systems program at ATCC. Dr. Lucchesi received her PhD in Cellular and Molecular Biology from the University of Campinas in Brazil and has over 20 years of experience in Tissue Engineering and Organ-on-Chip technology. In her current role, Dr. Lucchesi leads the MPS program bringing new capabilities in the use of advanced 3D models and developing existing and new content to be applied in state-of-art technologies.
Explore our related resources
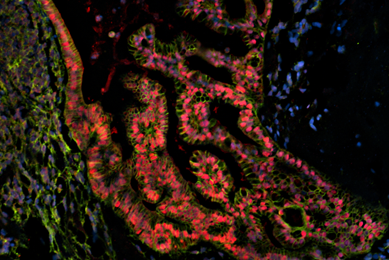
Organoids
Patient-derived organoids are authenticated cell models paired with genomic and phenotypic data. Organoids are available from the Human Cancer Models Initiative (HCMI) and contribute to valuable and reproducible research.
More
Toxicology
ATCC provides the tools you need to explore lung, skin, cardiovascular, gastro-enteric, liver, kidney, and neural toxicity. Our cells, media, and reagents help in toxicology research to identify responses to environmental toxins or to screen pharmaceutical compounds.
More
Cancer Research
Fighting cancer requires painstaking research and development. Scientists need materials and standards for drug screening, tumor mechanisms, cancer immunology, and cancer diagnostics. ATCC has research models such as organoids, conditionally reprogrammed cells, luciferase expressing reporter cell lines, isogenic CRISPR/Cas9 genome-edited cell lines, and epithelial-mesenchymal transition reporter cell lines.
MoreReferences
- Wikswo JP. The relevance and potential roles of microphysiological systems in biology and medicine. Exp Biol Med (Maywood) 239(9): 1061-1072, 2014. PubMed: 25187571
- Marx U, et al. Biology-inspired microphysiological systems to advance patient benefit and animal welfare in drug development. ALTEX 37(3 ): 365-394, 2020. PubMed: 32113184
- Ewart L, et al. Navigating tissue chips from development to dissemination: A pharmaceutical industry perspective. Exp Biol Med (Maywood) 242 (16): 1579-1585, 2017. PubMed: 28622731
- Vatine GD, et al. Human iPSC-Derived Blood-Brain Barrier Chips Enable Disease Modeling and Personalized Medicine Applications. Cell Stem Cell 24(6): 995-1005.e6, 2019. PubMed: 31173718
- Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat biotechnol 32(8): 760-772, 2014. PubMed: 25093883
- Ewart L, et al. Performance assessment and economic analysis of a human Liver-Chip for predictive toxicology. Commun Med (Lond) 2(1): 154, 2022. PubMed: 36473994
- Ribeiro AJS, et al. Liver Microphysiological Systems for Predicting and Evaluating Drug Effects. Clin Parmacol Ther 106(1): 139-147, 2019. PubMed: 30993668
- Fitzpatrick S, Sprando R. Advancing Regulatory Science Through Innovation: In Vitro Microphysiological Systems. Cell Mol Gastroenterol Hepatol 7(1): 239-240, 2019. PubMed: 30585159
- Kasendra M, et al. Duodenum Intestine-Chip for preclinical drug assessment in a human relevant model. ELife 9:e50135, 2020. PubMed: 31933478
- Apostolou A, et al. A Novel Microphysiological Colon Platform to Decipher Mechanisms Driving Human Intestinal Permeability. Cell Mol Gastroenterol Hepatol 12(5): 1719-1741, 2021. PubMed: 34284165
- Sun W, et al. Organ-on-a-Chip for Cancer and Immune Organs Modeling. Adv Healthc Mater 8(15): e1801363, 2019. PubMed: 31393091
- Smirnova L, et al. Organoid intelligence (OI) - The ultimate functionality of a brain microphysiological system. ALTEX 40(2): 191-203, 2023. PubMed: 37009773
- Ghosheh M, et al. Electro-metabolic coupling in multi-chambered vascularized human cardiac organoids. Nat Biomed Eng 7(11): 1493-1513, 2023. PubMed: 37550423
- Nawroth JC, et al. A Microengineered Airway Lung Chip Models Key Features of Viral-induced Exacerbation of Asthma. Am J Respir Cell Mol Biol 63(5): 591-600, 2020. PubMed: 32706623
- Ingber DE. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat Rev Genet 23(8): 467-491, 2022. PubMed: 35338360