hTERT-immortalized Neonatal Melanocytes – an Advanced In Vitro Cell-based Model for Pigmentation and Toxicity Studies
SOT 61th Annual Meeting and ToxExpo
San Diego, California, United States
March 28, 2022Abstract
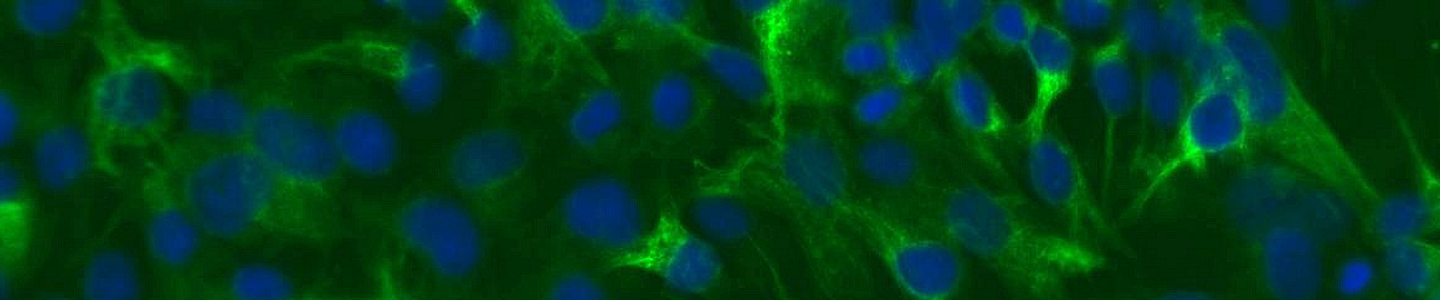
Pigmentation involves two main steps. First, melanocytes perform a myriad of complex biochemical and physiological steps to produce, package, and exocytose melanin containing melanosomes. Secondly, the melanosomes are taken up by neighboring keratinocytes where the stored melanin protects underlying tissues from damaging UV radiation. This process of pigmentation involves a complex interplay between genetic, endocrine, and environmental factors. Primary cells offer one model system to study pigmentation and dermal agents that may disrupt the melanogenesis; however, they are hindered somewhat by donor-to-donor variability and limited lifespan. Here, we created an immortalized melanocyte cell model—hTERT neonatal melanocytes—by retroviral transduction of human telomerase (hTERT) into primary cells. In addition to enhanced longevity (up to 35 doublings), physiologic marker expression (tyrosinase positive, fibroblast marker negative), and the ability to create melanosomes in 3D organotypic co-cultures, the cell line also showed expected levels of responses to several stimulators and inhibitors of melanogenesis. The inhibitors hydroquinone and kojic acid showed dose-dependent decreases in melanin content and the stimulators stem cell factor and latanoprost showed a muted response. In summary, immortalized melanocytes provided a versatile in vitro cell model for the study of skin toxicology and melanogenesis regulation.
Download the poster to explore the use of hTERT-immortalized neonatal melanocytes as a model for pigmentation and toxicity studies
DownloadWatch the poster presentation
Presenter
Diana Douglas, BS
Lead Biologist, ATCC
Diana Douglas is a Lead Biologist at ATCC. For the last nine years, she has focused her research on the development of advanced biological models including the use of CRISPR/Cas9 gene-editing technology. Previously, Ms. Douglas worked at the Baker Institute for Animal Health at Cornell University and the Dalton Cardiovascular Research Center at the University of Missouri, where her research focused on the mechanisms of necrotic cell death in heart disease. Ms. Douglas attended Truman State University where she obtained a Bachelor of Science in Biology.
Toxicology testing products
ATCC knows that worthwhile science takes time, especially in the toxicology, pre-clinical stages of drug development. It is critical that the standards and model organisms used in toxicological testing are reliable and authenticated. We can help streamline your research by providing the most authenticated, advanced, and functional models available. Let ATCC revolutionize and accelerate your toxicology studies in every phase of the research and testing process.
ATCC provides the cells, media, and reagents needed to explore each step of the in vitro preclinical testing process—from modeling, screening, and characterization to exploratory toxicology to pharmacokinetics and metabolism. We provide renal, neural, airway, and skin models for such applications as high-content screening, 3D culture, spheroid culture, permeability assays, metabolic stability and survival studies, transport activity measurement, and more.
Explore toxicology tools