Immune Checkpoint Reporter Cell Lines Based on the Protein Profiling of ATCC Cell Lines for Cancer Immunotherapy Drug Screening
AACR Annual Meeting 2023
Orlando, Florida, United States
April 19, 2023Abstract
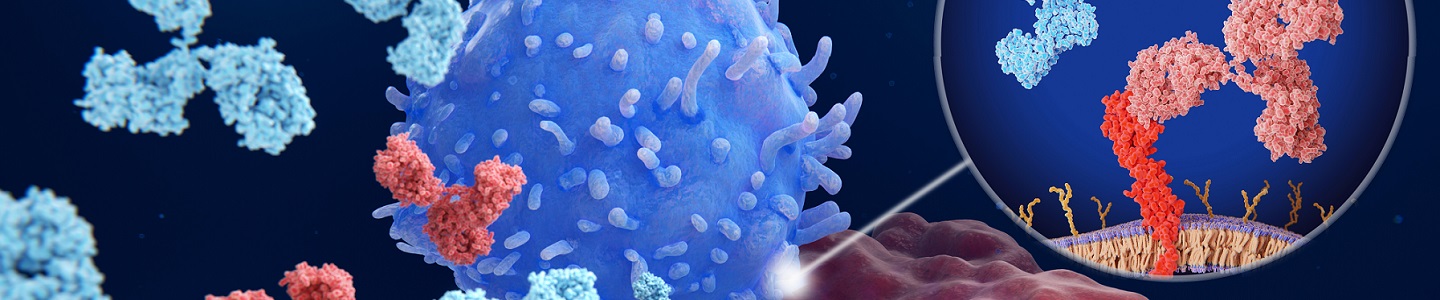
The success of immune checkpoint inhibitors in the treatment of various types of cancers and their continued growth in the market have driven burgeoning interests in developing more drugs in this category. However, the intrinsic complexity of the immunological models and the variable drug responses among different cancer types have become the most prominent challenges. To facilitate large-scale research projects and drug discovery of immune checkpoint inhibitors, we conducted a comprehensive protein profiling of ATCC’s vast portfolio of human tumor and immune cell lines for several established and novel immune checkpoint molecules. Based on this protein profiling data, we generated immune checkpoint reporter cancer cell lines with high expression of endogenous immune checkpoint molecule ligands (PD-L1, CD155, and B7-H3). The reporter system contains a gamma interferon activation site (GAS)-response element upstream of the luciferase gene, preventing luciferase expression when the immune checkpoint molecule ligand binds to its corresponding receptor that suppresses T cell–mediated antitumor activity. In the presence of a relevant immune checkpoint inhibitor, a luciferase expression-based bioluminescent signal is produced, which can be readily detected and quantitated to evaluate the efficacy, potency, and dynamics of the inhibitor. Our data showed that the bioluminescence in the reporter cancer cells increased approximately 100-250 folds in a dose-dependent manner in response to interferon gamma stimulation, which mimics the signaling from activated CD8+ cytotoxic T cells. The bioluminescence increased approximately 50-100 folds in response to CD8+ primary T cell–conditioned media stimulation. In particular, we observed up to 5-fold increase in luciferase signal from our PD-L1 reporter cell line in response to co-culture with CD8+ primary T cells in the presence of an anti-PD-L1 blocking antibody in a dose-dependent manner. The luciferase expression and endogenous immune checkpoint molecule ligand expression were well maintained after the cell lines had reached >30 population doubling level. These results highlight the robustness and responsiveness of the reporter system for the assessment of T cell–mediated immune responses triggered by checkpoint inhibitors. These immune checkpoint reporter cancer cell lines yield exceptional in vitro and ex vivo assay sensitivity and reproducibility, while simplify the complex immunological model by providing physiologically relevant expression of immune checkpoint molecule ligands, in comparison to similar assays with an artificial checkpoint ligand overexpression system.
Download the poster to explore the development and application of immune checkpoint reporter cells
DownloadWatch the poster presentation
Presenter
Hyeyoun Chang, PhD
Scientist, ATCC
Hyeyoun Chang, PhD, is a Scientist in the Immuno-oncology group of the R&D department at ATCC. She has extensive experience in the fields of biomedical engineering and cancer biology that focuses on drug delivery, intracellular signaling, and gene therapy. Prior to joining ATCC, Dr. Chang received her PhD in biomedical engineering from Korea University of Science and Technology and completed her postdoctoral training at Dana-Farber Cancer Institute/ Harvard Medical School.
