Molecular diagnostic assays are powerful tools for the rapid detection and identification of clinically relevant pathogens. That's why it is essential that they are properly validated to ensure accurate results and uncompromised data.
As your end-to-end partner for the development and control of diagnostic assays, ATCC supports each stage of your workflow by providing authenticated live strains, quantitative molecular standards, and next-generation sequencing standards. Explore our resources below to see how we can collaborate on your next assay.
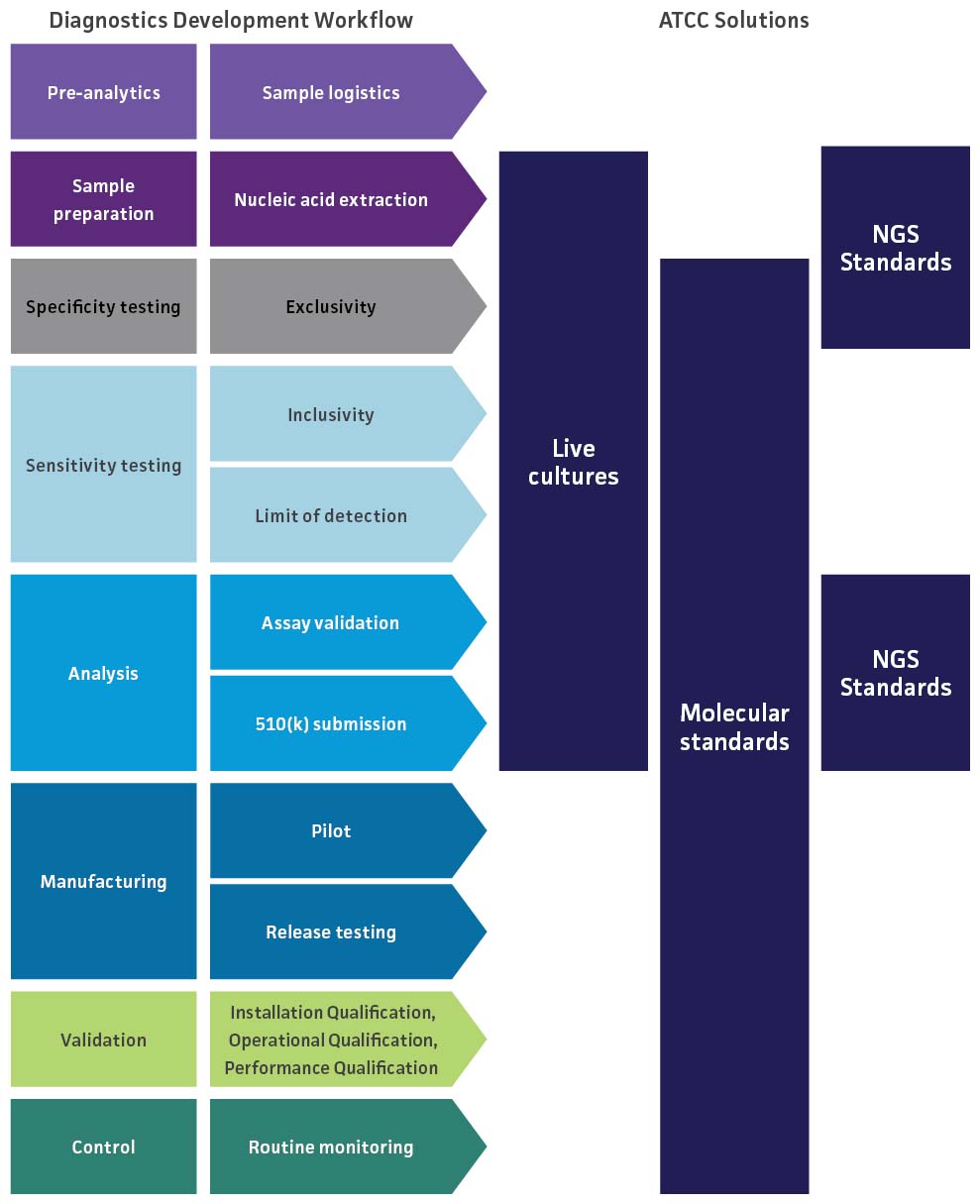
Find solutions for each stage of your workflow
Explore assay development resources for infectious diseases
SARS-CoV-2 molecular diagnostics development
For SARS-CoV-2 molecular diagnostics manufacturers moving from emergency use authorization (EUA) to 510(k) premarket submission to the US Food & Drug Administration (FDA), robust testing must be performed to demonstrate that the device is safe and effective as well as substantially equivalent to legally marketed devices. To support these validation studies, ATCC provides an extensive array of authenticated and clinically relevant strains and genomic and synthetic nucleic acids for evaluating limit of detection, inclusivity, and cross-reactivity.
Develop your SARS-CoV-2 AssayMonkeypox virus molecular diagnostics development
Accurate and rapid diagnosis of mpox is critical for timely healthcare and tracking of transmission. To support the need for increased monitoring, ATCC provides a variety of orthopoxviruses and nucleic acids that support assay development and validation. We have also developed a research use only (RUO) quantitative synthetic monkeypox virus DNA preparation with a sequence design based on several published assays.
Develop your mpox assay