Advanced 2D and 3D Cardiomyocyte-based Models for Use in Drug Discovery
Microphysiological Systems World Summit
Seattle, Washington, United States
June 13, 2024Abstract
Cardiovascular disease (CVD) is the leading cause of mortality worldwide, imposing considerable health and economic burden. The lack of relevant in vitro models hinders the development of cardiovascular drugs or the prediction of cardiotoxicity from new drug candidates. Although human induced pluripotent stem cell (hiPSC)-derived cardiomyocytes (CMs) provide a promising source of CMs, with the current technology these in vitro differentiated hiPSC-CMs often fail to recapitulate the phenotype and physiologically relevant functionality. The maturation severely impacts the use of iPSC-CMs in in vitro modeling for pathological, pharmacological, or therapeutic purposes since the electrophysiology and mechanical function and metabolism are suboptimal. Various studies have demonstrated the use of electrical or mechanical stimulation, as well as artificial tissue scaffolds, to promote the maturation of hiPSC-CMs in vitro. However, these techniques often require months and have low throughput.
To address these issues, we have focused our work on harnessing the power of the ATCC Maturation Reagent (AMR) to effectively propel the maturation of hiPSC-CMs in 2-D monolayer culture. This method paves the way for the mass production of high-quality and mature hiPSC-CMs that exhibit a mature phenotype with regard to morphology, structure, gene expression, metabolism, calcium handling, and contractile performance.
In addition, we are integrating this enhanced cardiomyocyte function methodology with the 3-D technology Robot-Directed Organoid Deposition (RODEO) to further advance the creation of more in-vivo–like cardiac models. The combined power of these technologies holds the promise to revolutionize the creation of in vitro cardiac tissue that truly mimic the structural and biochemical properties of the cardiac environment. The resulting vascularized multichambered cardiac organoids are highly similar to adult cardiac muscle transcriptionally and respond to drugs that mimic physiological conditions. This disruptive high-throughput technology underscores the utility of microphysiological systems for use in the drug discovery process and improving clinical success rates.
Download the poster to learn about the development of vascularized multichambered cardiac organoids.
DownloadPresenter
Carolina Lucchesi, PhD
Principal Scientist, BioNexus, ATCC
Carolina Lucchesi is BioNexus Foundation Principal Scientist leading the Microphysiological Systems program at ATCC. Dr. Lucchesi received her PhD in Cellular and Molecular Biology from the University of Campinas in Brazil and has over 20 years of experience in Tissue Engineering and Organ-on-Chip technology. In her current role, Dr. Lucchesi leads the MPS program bringing new capabilities in the use of advanced 3D models and developing existing and new content to be applied in state-of-art technologies.
Featured resources
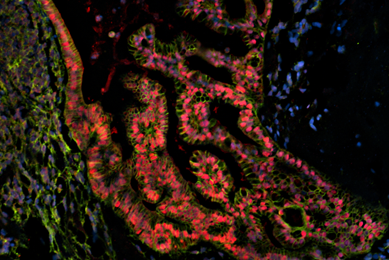
Organoids
Patient-derived organoids are authenticated cell models paired with genomic and phenotypic data. Organoids are available from the Human Cancer Models Initiative (HCMI) and contribute to valuable and reproducible research.
More
Human Induced Pluripotent Stem Cells
Using ATCC’s complete culture systems, researchers can cultivate standardized, quality controlled, and highly characterized human iPSCs lines.
MoreMicrophysiological Systems
Learn how microphysiological systems are transforming drug development and our understanding of human diseases.
More